346
AFRICA
CARDIOVASCULAR JOURNAL OF AFRICA • Advance Online Publication, November 2011
STEMI or unstable angina.
Key exclusion criteria were patients
with a history of stroke (ischaemic stroke
or transient ischaemic events despite
being on aspirin and a thienopyridine),
previous gastrointestinal or intracranial
bleeding, or evidence of bleeding seque-
lae such as a platelet count of less than
90 000 per mm
3
, a haemoglobin level
lower than 10 g/dl and a low creatinine
clearance rate of less than 30 ml/min on
screening for possible study entry.
‘The reason for excluding patients who
had had a stroke derives from our knowl-
edge that if you have had a stroke, even
dual anti-platelet therapy may not be opti-
mal. To support this approach, a few stroke
patients did slip into theATLAS study and
their outcome was poor’, Dr Gibson noted
during the post-clinical trial interviews.
‘However, in patients who did experi-
ence a stroke during this TIMI trial, those
on rivaroxaban had a better neurological
recovery. This may well be due to the fact
that thrombin is known to increase apop-
tosis in the neural system and inhibition
of thrombin generation can reduce neural
apoptosis, offering a useful hypothesis for
some of the benefits on stroke severity
seen in this trial’, Dr Gibson explained.
The ATLAS ACS 2-TIMI 51 trial was
a phase-three trial initiated with an oral
dosage of either 2.5 or 5 mg rivaroxaban
twice daily (bid). This dosage was based
on a previous phase-two dose-finding
trial. Patients received aspirin, a thieno-
pyridine and placebo, or one of the two
rivaroxaban doses on an average of 4.7
days after revascularisation procedures
had been done and the patient was stable.
Importantly patients did as well on the
lower dose, with less bleeding than on
the higher dose. There was an increase
in major bleeding rates not related to
coronary bypass grafting and intracranial
bleeding without a significant increase
in fatal bleeding or adverse events in
patients receiving rivaroxaban compared
to standard therapy.
Expert opinion
Prof Sylvia Haas, Technical University,
Munich, Germany, a well-known expert
from Munich and frequent visitor to
South Africa commented on the impor-
tance of this study for clinical practice.
The results of ATLAS ACS 2-TIMI
51 have the potential to lead into a new
era in secondary prevention of thrombo-
embolic complications after ACS. This
landmark study aimed to lower cardiovas-
cular events in patients with recent ACS
compared to standard care and this has
been successfully achieved for both doses
of the oral factor Xa inhibitor rivaroxaban
tested, and for each dose alone.
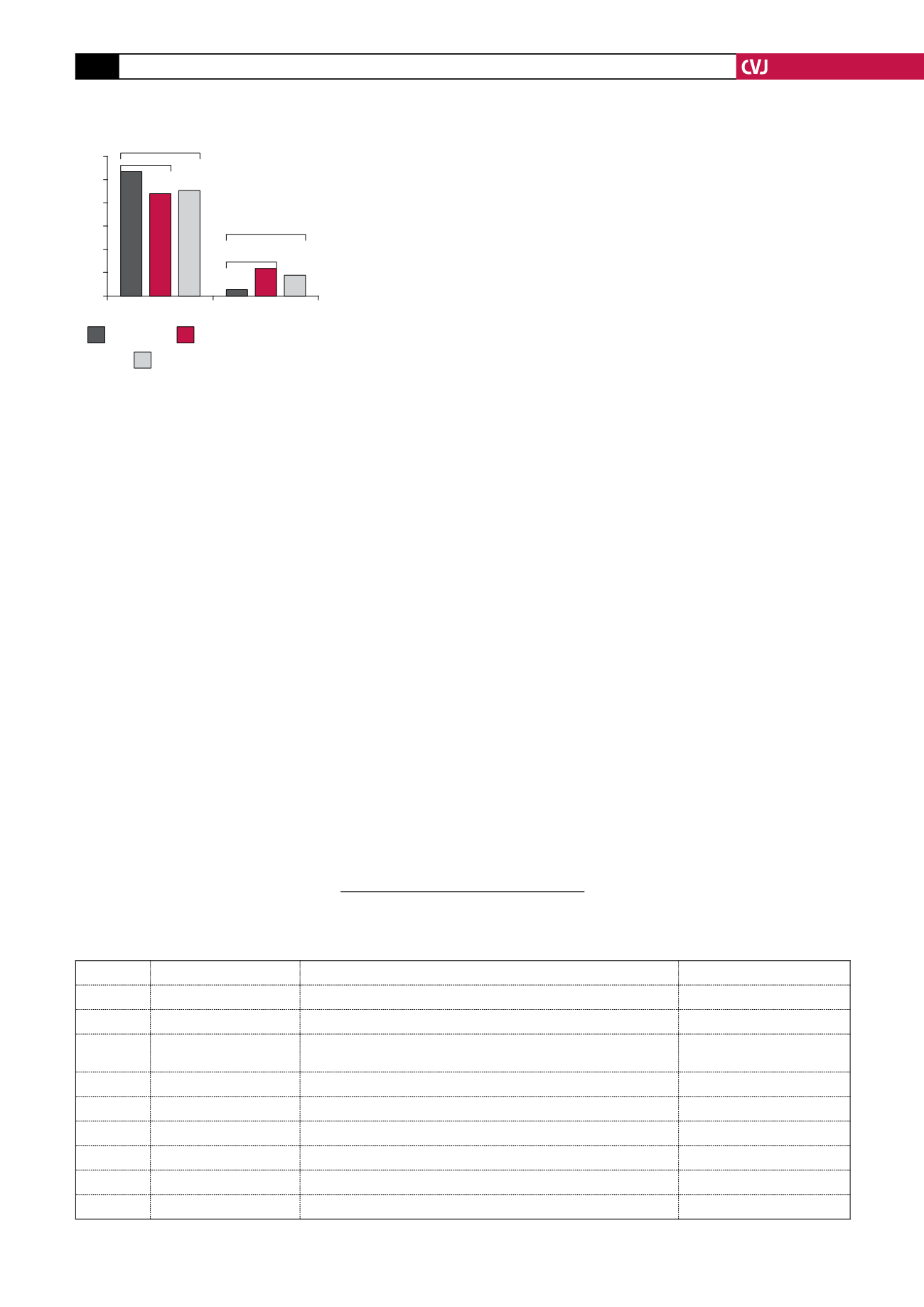
A cumulative incidence of 10.7%
for the combined endpoint, consist-
ing of cardiovascular death, myocardial
infarction (MI) and stroke, was seen in
patients randomised to placebo and this
was reduced to 8.9% for both rivaroxa-
ban groups combined. In patients treated
with the higher dose of rivaroxaban of 5
mg bid, this endpoint was significantly
reduced to 8.8%, and for patients treated
with 2.5 mg bid to 9.1%, which was also
statistically significant. There were also
reductions in rates of death from both
cardiovascular causes and any cause for
the 2.5-mg dose but not for the 5-mg
dose.
As expected, the bleeding rates were
higher for the patients receiving the
combination of anticoagulation and anti-
platelet therapy. This effect was dose
related, i.e. bleeding rates were lower
in the 2.5- than in the 5.0-mg group.
Although the rate of intracranial bleeding
was higher than with placebo, there was
no increase in fatal bleeding events.
In conclusion, rivaroxaban is the first
new oral anticoagulant to demonstrate a
clinically relevant benefit in ACS.
1.
FDA Announcement,11 November 2011.
2.
Mega JL, Braunwald E, Wiviott SD, Bassand
JP. Rivaroxaban in patients with recent
acute coronary syndrome. DOI:10:1056/
NEJMoa1112277.13 November 2011.11.13.
3.
Einstein investigators. Oral rivaroxaban for
symptomatic venous thromboembolism.
N
Engl J Med
2010;
363
: 2499–2510.
Fig. 1. Efficacy and safety endpoints
for three treatment arms
12
10
8
6
4
2
0
Cumulative incidence (%)
HR 0.84,
p
=
0.020
CV death/MI/stroke Major bleeding
Placebo
5.0 mg rivaroxaban
2.5 mg rivaroxaban
HR 0.85,
p
=
0.028
HR 3.46,
p
=
0.001
HR 4.47,
p
<
0.001
Cardiovascular diary for 2012 congresses
DATE
PLACE
CONFERENCE
WEBSITE
1–3 February New Orleans, USA
Internatioanl Stroke Conference
18 February Frankfurt, Germany
TrenD 2012 – Transcatheter renal denervation
3–5 March
Southern Sun Cape Sun,
Cape Town, South Africa
17th Biennial Congress of the SA Hypertension Society Congress (SAHS)
18–21 April
Dubai, United Arab Emirates World Congress of Cardiology
17–20 May Berlin, Germany
Congress on cardiac problems in pregnancy (CPP 2012)
27 June
Frankfurt, Germany
ICI 2012 – Imaging in cardiovascular interventions
28 June
Frankfurt, Germany
CSI 2012 – Catheter interventions in congenital and structural heart disease
19–22 July Sun City, South Africa
13th Annual SA Heart Congress
25–29 August Munich, Germany
2012 ESC – European Society of Cardiology Congress


