CARDIOVASCULAR JOURNAL OF AFRICA • Vol 23, No 7, August 2012
366
AFRICA
management and outcomes of patients admitted to hospital for
acute coronary syndromes in South Africa.
The aim of the multinational observational ACCESS
(Acute Coronary Events – a Multinational Survey of Current
Management Strategies) registry was to gain insights into
the descriptive epidemiology, current practice patterns, and
one-year outcomes of patients hospitalised with acute coronary
syndrome (ACS), whether this be unstable angina (UA)/non-ST-
segment elevation acute coronary syndrome (NSTE-ACS)
or ST-segment elevation myocardial infarction (STEMI), in
developing countries in Africa, Latin America and the Middle
East, excluding countries from Europe and North America.
The complete ACCESS study design, methods and results
have previously been published.
9
The following report is of the
data applicable to the South African patient cohort, and how
these compare with the data from the ACCESS study as a whole.
Methods
ACCESS was a prospective, observational, multinational registry
in patients hospitalised for an acute coronary event. Patients were
enrolled at 134 sites in 19 countries in North Africa (Algeria,
Morocco, Tunisia), South Africa, Latin America (Argentina,
Brazil, Colombia, Dominican Republic, Ecuador, Guatemala,
Mexico, Venezuela) and the Middle East (Egypt, Iran, Jordan,
Kuwait, Lebanon, Saudi Arabia, United Arab Emirates).
The ACCESS registry was conducted in accordance with the
Guidelines for Good Clinical Practice and under the leadership
of a scientific advisory board (the ACCESS steering committee).
Each participating country had the responsibility of ensuring
locally that all necessary regulatory submissions were performed
in accordance with local regulations, including data-protection
regulations. Local institutional review boards or independent
ethics committees approved the study and all patients provided
informed consent to participate.
Selection of physicians was determined at the country level;
29 sites participated in South Africa. The aim was to enroll
approximately 25 patients per site.
Patients aged 21 years and older who were admitted alive to
hospital with an ACS and provided signed, informed consent
were eligible to participate in this all-comers registry. Patients
with symptoms precipitated by a secondary co-morbidity (e.g.
anaemia, heart failure or non-cardiac trauma) and patients
participating in concomitant clinical trials were excluded from
the study. Eligible patients who agreed to participate were
recruited consecutively to avoid patient selection bias.
To be enrolled, patients had to present with ischaemic symp-
toms of ACS within 24 hours of hospital presentation and have
at least one of the following: (1) electrocardiographic changes
[transient ST-segment elevation
≥
1 mm, ST-segment depression
≥
1 mm, new T-wave inversion
≥
1 mm, pseudo-normalisation
of previously inverted T waves, new Q waves (one-third of the
height of the R wave or
>
0.04 s), new R wave
>
S wave in lead
V1, or new left bundle branch block]; (2) documentation of
coronary artery disease [history of myocardial infarction (MI),
angina, congestive heart failure believed to be due to ischaemia
or resuscitated sudden cardiac death, history of or new positive
stress test with imaging, previous or new cardiac catheterisation
documenting coronary artery disease, prior or new percutaneous
coronary intervention (PCI) or coronary artery bypass surgery
(CABG)]; and (3) an increase in a cardiac biochemical marker
of myocardial necrosis (troponin or CK-MB).
The data were recorded prospectively on admission to hospital
(baseline), at discharge, and at 6
±
1 months’ and 12
±
1 months’
follow-up visits. Data were collected via telephonic interviews
for patients who did not attend the follow-up appointments.
In each country, 10% of sites that enrolled at least one patient
were randomly selected to undergo a site visit and data audit. At
each of these sites, 100% of case report forms for all enrolled
patients were monitored for source documentation and accuracy.
The primary endpoint was all-cause death at one year from
initial hospitalisation. Secondary endpoints (one year from initial
hospitalisation) included cardiovascular death, cardiovascular
death and non-fatal MI, non-fatal stroke, non-fatal MI, the
combined endpoint of cardiovascular death, stroke or myocardial
infarction and re-hospitalisation for ischaemic events, the
composite endpoint in each country, and bleeding episodes.
All-cause death at 30 days was also recorded.
Results
Recruitment of patients took place between January 2007 and
January 2008. Of the 39 participating doctors in the 29 South
African recruiting sites, 26 identified themselves as cardiologists,
of whom 10 (38.5%) were interventional cardiologists. All major
geographic areas in South Africa were involved in recruitment
of registry patients. A list of the participating doctors is given at
the end of the article.
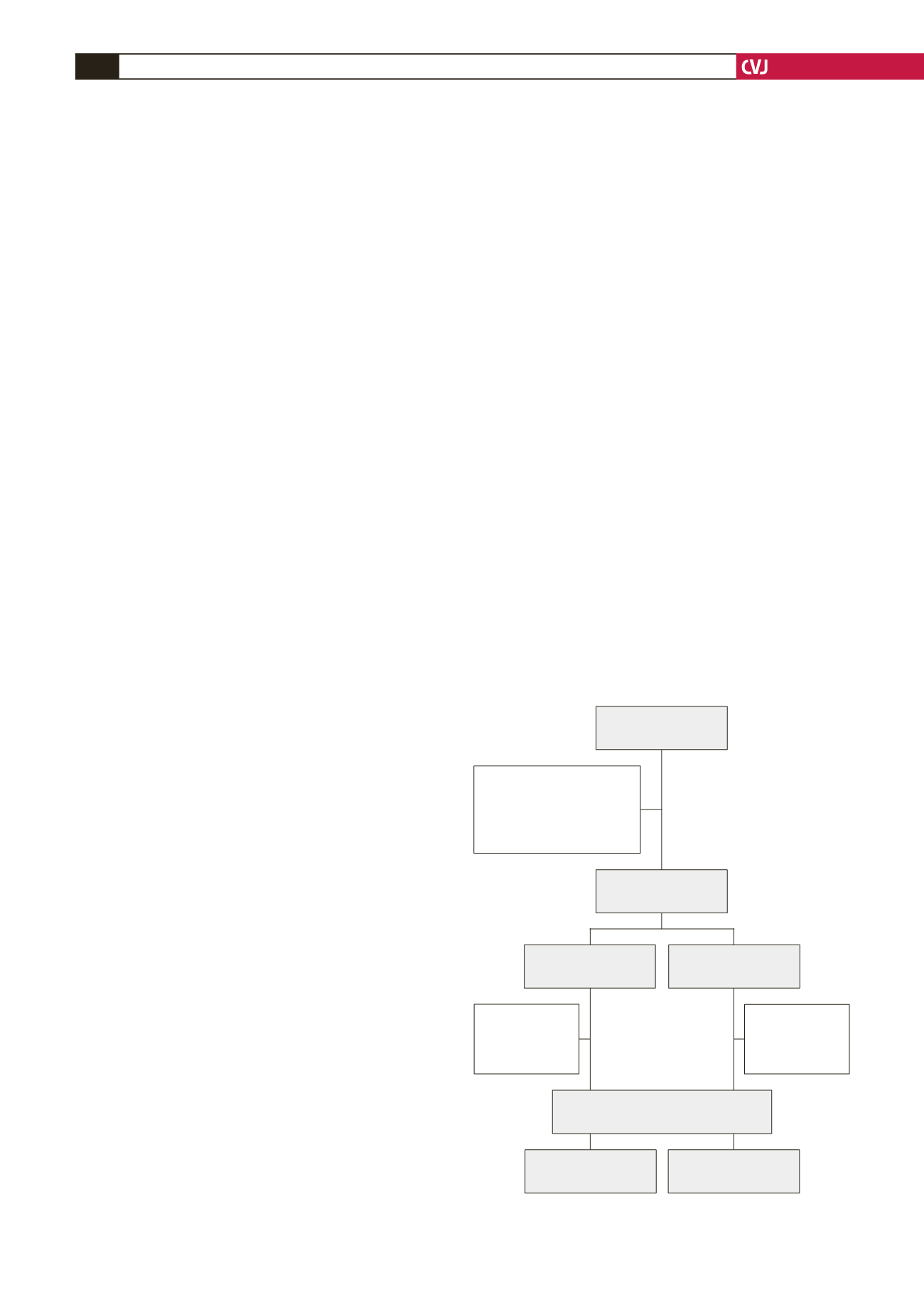
A total of 642 patients were enrolled in South Africa, 5.3%
of the 12 068 enrolled in the entire ACCESS registry. In the
Fig. 1. Study flow chart.
Patients enrolled
(
n
=
642)
Exclusions:
Other cardiac diagnosis
(
n
=
16)
Non-cardiac diagnosis
(
n
=
11)
NSTE-ACS
(
n
=
328)
STEMI
(
n
=
220)
ACS patients
(
n
=
615)
NSTE-ACS
(
n
=
362)
STEMI
(
n
=
253)
Exclusions:
Died (
n
=
18)
Lost to follow-
up (
n
=
16)
Exclusions:
Died (
n
=
17)
Lost to follow-
up (
n
=
16)
Patients with one-year follow up
(
n
=
548)


