CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 6, July 2013
AFRICA
203
type one (ADO I) (St Jude Medical, Cardiovascular Division, St
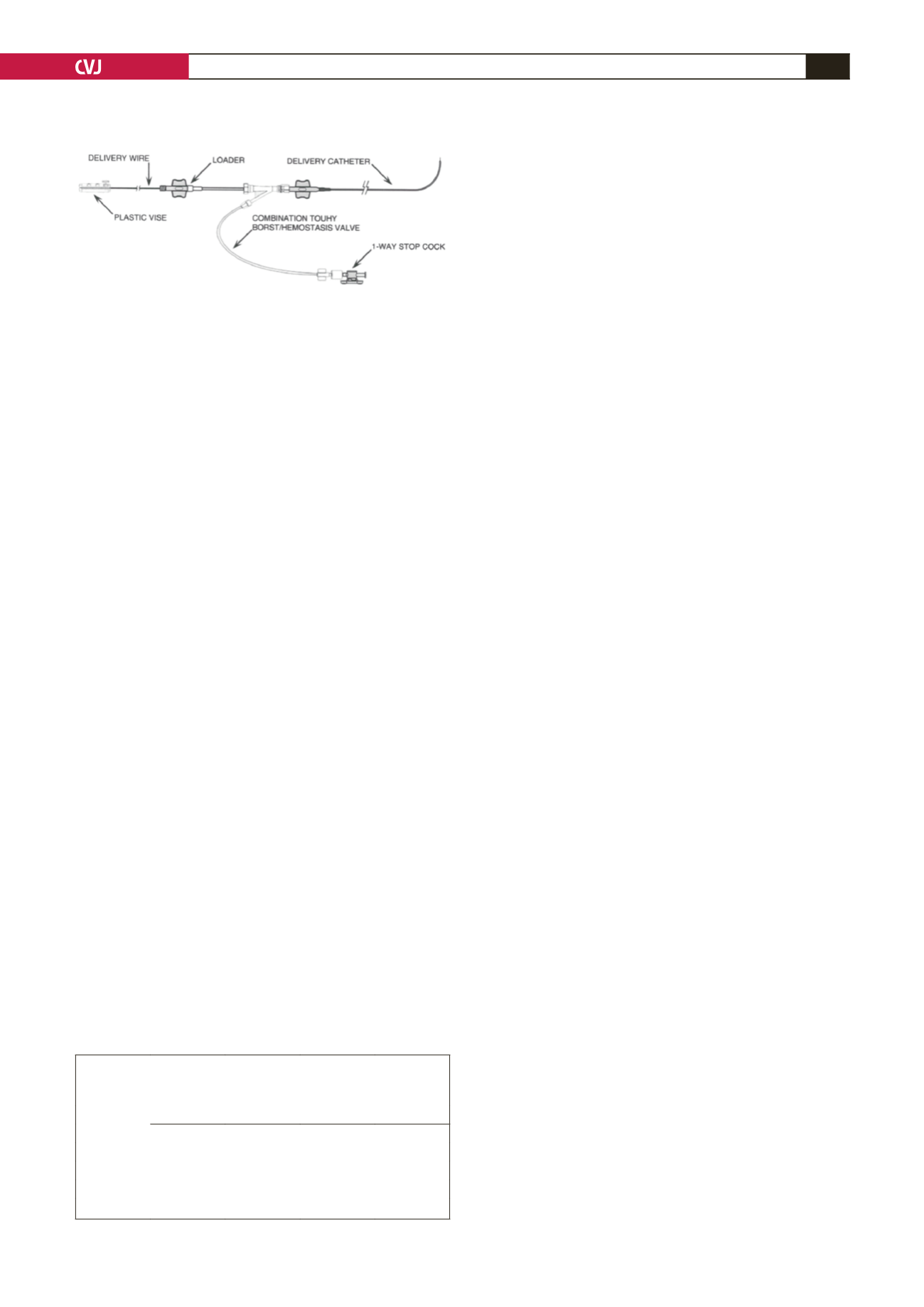
Paul, MN). The delivery system has delivery sheaths of 4- and
5-F in size, with a length of either 60 or 80 cm; delivery wire
with a screw mechanism to attach the device; device loader;
Y-connector and a plastic vise.
Informed consent is obtained before attempting percutaneous
ductal closure. Under conscious sedation, the patient is scrubbed
and draped to ensure a sterile environment. Femoral arterial and
venous access is achieved using standard vascular-access short
sheaths. About 50 IU/kg of heparin are then given through the
arterial sheath. Descending aortography in the straight lateral
view is performed.
The size and shape (type) of the PDA using the Krichenko
classification are determined.
18
Standard left and right cardiac
catheterisation procedures are then performed. Calculations to
ascertain the extent of left-to-right (or right-to-left) shunting,
pulmonary vascular and systemic vascular resistances are done.
Following angiography and haemodynamic data, the decision
whether or not to close the PDA is then made. If the PDA
is amenable to percutaneous closure based on the size and
length of the duct, an appropriate device is selected using
the manufacturer’s device selection table (St Jude Medical,
Cardiovascular Division, St Paul, MN) as a guide (Table 1). The
delivery system is then flushed using heparinised saline.
A 0.035-inch guide wire is passed across the PDA using an
end-hole catheter, either in an anterograde fashion through the
pulmonary side or in a retrograde manner via the aortic route.
The ADO II delivery sheath is passed across the PDA over the
guide wire. Blood is allowed to flow from the back of the sheath
to purge all air from the system. The delivery wire is passed
through the loader. The device is attached to the delivery wire
using a screw mechanism.
Under water, the device is retrieved into the loader so that
its distal radiopague end is at the tip of the loader. The loader is
firmly introduced into the delivery sheath. Under fluoroscopy,
the device is advanced into the sheath using the delivery wire
until it reaches the tip of the delivery sheath. At this stage the
whole assembly is repositioned until the operator is satisfied, to
deploy the distal disk.
Once the distal disk is well positioned and conforms to the
vessel wall, the middle lobe is deployed in the duct with the
proximal disk deployed on the other end of the PDA.Angiography
may be performed at any stage of device deployment using the
Y-connector and an angiographic catheter to check for device
positioning in the duct, pulmonary artery or aorta. The device is
released or retrieved as the operator deems fit.
The patient receives an intravenous antibiotic and may receive
prophylaxis for infective endocarditis for six months. The patient
is followed up at one day, one month, three months, six months,
one year and two years following transcatheter closure of the
PDA, using this device, to look for complications that may arise
from the catheterisation procedure or the device itself. After two
years’ follow up, patients are discharged.
Complications relating to closure of PDA in our patients,
including aortic and (left) pulmonary obstruction, and device
embolisation are documented. Short-term outcomes are also
reported.
Statistical analysis
Values were reported as mean
±
standard deviation (SD) and
median (range). Statistical significance was not required, as data
comparison was not done.
Results
Between May 2009 and July 2012, 36 patients were selected
for percutaneous closure of the PDA using the Amplatzer duct
occluder II. Their median age was 16.5 months (range: 2–233),
with a median weight of 8 kg (range: 3.9–39.2), and a median
height of 75 cm (range: 55–166). There were 21 females and 15
males. Patients’ basic characteristics and haemodynamic data are
presented in Table 2.
The mean pulmonary artery pressure was 24.4 (SD:
±
10.4)
mmHg, while the mean systolic pulmonary artery pressure was
34.8 (SD:
±
14.5) mmHg (Table 2). The Qp:Qs ratio was 2.25
(SD:
±
1.97), while the Rp mean was 1.87 (SD:
±
1.28) Wood
units.
Table 3 shows angiographic data and outcomes in ductal
closure using the ADO II. According to the Krichenko
classification, 16 PDAs were type A (conical), four were type
B (A-P window like), five were type C (tubular and more than
3 mm in length), two were type D (complex, with more than
one constriction site), and nine were type E (long with sudden
tapering at the pulmonary end). In terms of size, the narrowest
mean ductal diameter (PDA size) was 2.74 (SD:
±
1.3) mm, with
a mean PDA length of 9.5 (SD
±
4.16) and mean aortic ampulla
of 9.46 (SD:
±
4.1) mm.
In terms of device choice, nine patients were closed using a
3
×
6-mm device, two with a 4
×
4-mm device, seven with a 4
×
6-mm device, four with a 5
×
6-mm device, one with a 6
×
4-mm
device, and 13 with a 6
×
6-mm device. Regarding the delivery
of the device, in 30 patients, the device was delivered through
the pulmonary artery, while in six it was in a retrograde fashion
through the aorta.
The exposure to radiation had a median of 20.2 minutes
(range of 7.1–88.7). In terms of closure rates, 33 patients
(91.67%) achieved complete closure by discharge (day one) and
Fig. 2. A picture of the Amplatzer duct occluder type
II TorqVue low-profile delivery system. (Figure used
with permission from St Jude Medical, Cardiovascular
Division, St Paul, MN).
TABLE 1. MANUFACTURER’S GUIDELINES REGARDING
ADO II DEVICE SIZE CHOICE IN RELATION TOTHE
PDA SIZEAND LENGTH
Ductal length
Ductal size
< 5 mm 5.1–8 mm 8.1–10 mm 10.1–11 mm
< 2.5 mm 3 × 4
3 × 6
4 × 6
5 × 6
2.5–3.5 mm 4 × 4
4 × 6
5 × 6
6 × 6
3.6–4.5 mm 5 × 4
5 × 6
5 × 6
6 × 6
4.6–5.5 mm 6 × 4
6 × 6
6 × 6
6 × 6


