
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 26, No 1, January/February 2015
6
AFRICA
As presented in Table 1, IMA rings were obtained from a
range of patients undergoing CABG surgery due to various
cardiovascular diseases. Although most of the associated risk
factors have been shown not to affect endothelium-dependent
contractile responses of the arteries from these patients,
20
and
both endothelium and smooth muscle are affected by risk factors,
we chose to use endothelium-independent relaxation protocols.
The finding of SNP-induced (endothelium-independent)
relaxation of the IMA rings indicated that possible injury to
the endothelium during harvesting and/or grafting does not
totally impair the relaxation capacity of this conduit artery. The
contractile functionality of coronary artery grafts has been a
topic of substantial interest and has been studied extensively in
different vessels, including human saphenous veins, radial artery
and IMA.
21-24
Leptin has been shown to cause endothelium-dependent
vasorelaxation of the peripheral arteries of experimental
animals.
25
Leptin has also been shown to exert an endothelium-
independent vasodilatory action in humans with coronary artery
disease.
26
Therefore, in addition to its central role in the regulation
of energy balance and metabolism, leptin has direct effects on
the blood vessels (atherogenic, thrombotic and angiogenic) of
both coronary and cerebral arteries, potentially contributing to
the progression of atherosclerosis in the coronary vessels.
27-29
Conclusion
By investigating the mechanism and effect of leptin on NE
pre-contracted IMA segments, a vessel commonly used for
CABG, our
in vitro
study has provided further pharmacological
evidence on the characteristics of this vessel. Leptin induced
direct vasodilatation of the IMA, and PKC was potentially a
sub-cellular mediator for the leptin-induced vasodilatation of
these arteries. Although the physiological function of leptin
100
80
60
40
20
0
–9
–8
–7
–6
–5
–4
Norepinephrine (log м)
Tension (%)
Control
Leptin (1
μ
M)
100
75
50
25
0
–9
–8
–7
–6
–5
–4
Norepinephrine (log м)
Tension (%)
PKC Inhibitor (10
μ
M)
Leptin (1
μ
M) + PKC Inhibitor (10
μ
M)
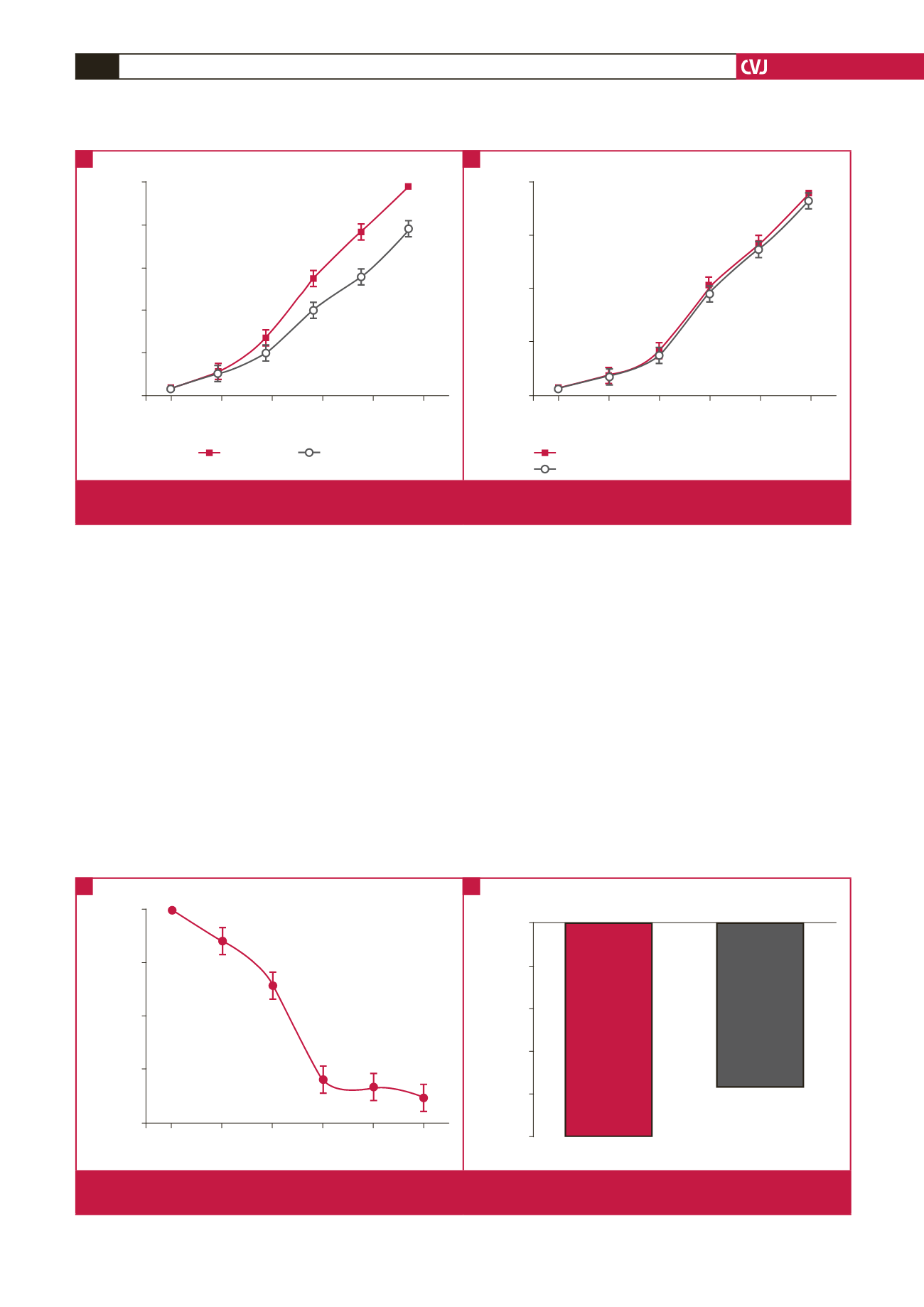
Fig 1.
Effects of leptin on sodium nitroprusside-induced relaxation response in norepinephrine pre-contracted human internal
mammary artery rings in the absence (A) and presence (B) of chlerythrine chloride (PKC inhibitor).
A
B
0
–20
–40
–60
–80
–9
–8
–7
–6
–5
–4
Sodium-nitropruside (log м)
Percantage pre-contraction
0
–20
–40
–60
–80
–100
Tension (%)
Control
Leptin (1
μ
M)
Fig 2.
Effects of leptin on sodium nitroprusside-induced relaxation in norepinephrine pre-contracted human internal mammary
artery rings. The magnitude of the relaxation response is expressed by tension bars.
A
B



















