
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 30, No 3, May/June 2019
182
AFRICA
(XO), endothelial nitric oxide synthase (eNOS) uncoupling,
NADPH oxidase and mitochondria. Ultimately the reunion
of all these events leads to peroxynitrate formation, lipid
peroxidation, protein modification, matrix metalloproteinase
(MMP) activation and DNA damage, contributing to endothelial
dysfunction.
24,25
The source of the complex mediators is within
the ischaemic trophoblastic cell, which filters into the maternal
and foetal circulations.
One of the main mechanisms of endothelial dysfunction
involves the release of soluble fms-like tyrosine kinase 1
(sFLT-1), an anti-angiogenic protein and inhibitor of vascular
endothelial growth factor (VEGF) that works by enhancing the
endothelial dysfunction already established by oxidative stress,
ROS and damage.
26
Immediately after placental reperfusion
injury, re-established blood flow releases cytokines, TNF-alpha,
interleukin-6, interleukin-10, C-reactive protein and damaging
levels of ROS such as superoxide in response to these events.
27
These complex mediators and the constant interaction and
interplay between the three components of pregnancy, that
is, mother, foetus and placenta, lead to the unified hypothesis
that may explain the development of both maternal and
foetal morbidity and/or mortality on a unitary basis in severe,
complicated pre-eclampsia.
Based on the data,
1-27
a unified theory of cardiac dysfunction in
pre-eclampsia in the materno-foetal complex could be proposed,
on the basis that both the maternal and foetal compartments are
exposed to similar haemodynamic challenges. Both maternal and
foetal compartments are flooded with anti-angiogenic substrates
and complex mediators from a chronically ischaemic placenta,
causing widespread vasoconstriction and endothelial cell damage.
This is augmented by substantial increases in catecholamine
secretion in the maternal compartment, resulting in changes in
maternal left ventricular filling and diastolic dysfunction, left
ventricular hypertrophy, increasing end-systolic and end-diastolic
left ventricular volumes and the precipitation of myocardial
ischaemia and arrhythmias with cardiac decompensation. A
similar pathophysiology in the foetus leads to diastolic dysfunction
(altered E/A ratios) and altered global cardiac function (as reflected
in abnormal myocardial performance indices). The response of
the maternal component in the pregnancy state is further modified
by pre-existing or subclinical, latent cardiovascular disease. These
findings fit well into a tri-aetiological/pathophysiological basis for
pre-eclampsia (Figs 1, 2):
•
a maternal component from potential or pre-existing cardio-
vascular disease, showing up as superimposed severe pre-
eclampsia (mother failing the cardiovascular stress of preg-
nancy)
•
a placental component from chronic utero-placental ischae-
mia due to placental maladaptation and lack of placenta
vascular transformation
•
a foetal component, which induces compensatory signalling
mechanisms in response to the chronic utero-placental ischae-
mia to improve placental circulation, and also exhibiting
cardiac haemodynamic changes in itself.
This integrated model proposes a holistic approach in the
evaluation of the cardiac status of the materno-foetal complex
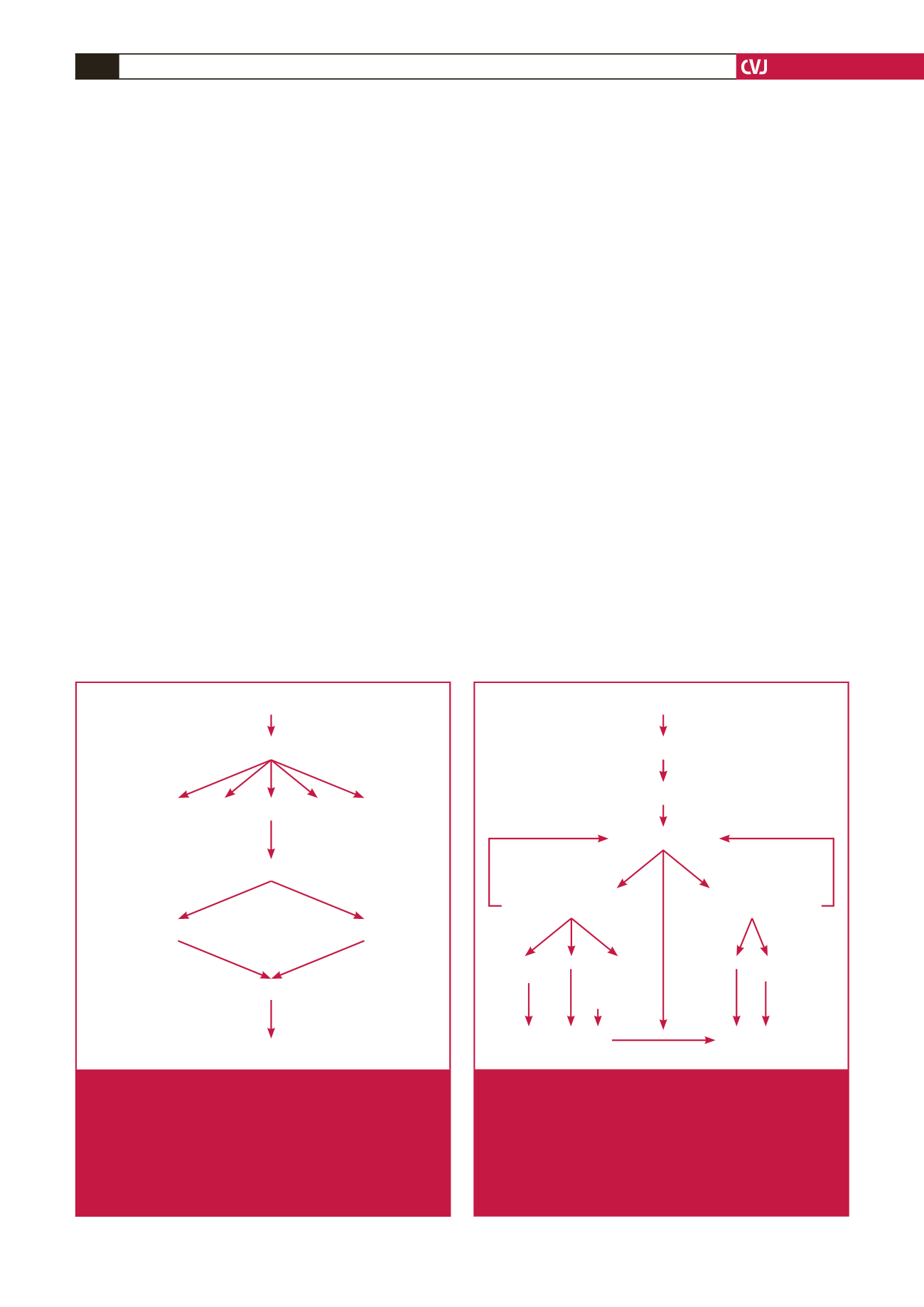
Cardiac dysfunction in materno-foetal complex
Placental maladaptation
Chronic utero-placental ischaemia
Anti-angiogenic
factors
(PLGF/VEGF)
(sFlt/endoglin)
Angiogenic
factors
cytokines
AT1-AA
STMBs
Widespread vasoconstriction + increased SVR
Maternal circulation
mirror
syndrome
Foetal circulation
Severe pre-eclampsia
foetal
signalling
mechnisms
Pre-existing/latent
+ cathecolamines
cardiovascular
dysfunction
Fig. 1.
Pathophysiological pathway to cardiac dysfunction in
the materno-foetal complex in severe pre-eclampsia,
showing the proposed interaction of the materno-
placental-foetal complex with each other. STMB:
syncytiotrophoblast microparticles, AT1-AA: circulating
auto-antibodies to angiotensin II AT-1 receptors, VEGF:
vascular endothelial growth factor, PLGF: placental
growth factor, sflt-1: soluble fms-like tyrosine kinase 1.
Placental ischaemia
Severe pre-eclampsia
Widespread vasoconstriction + SVR
Anti-angiogenic factors/angiogenic factors imbalance
Pre-existing/latent
cardiovascular disease
foetal signalling
mechanisms
Altered maternal
cardiac haemodynamics
Foetal cardiac
dysfunction
Pulmonary oedema in
severe pre-eclampsia
Adverse peri-natal
outcome
BNP
Diastolic
dysfunction
LVH LV-ESV +
LV-EDV
ventricular
arrythmias
MPI E/A
ratio
cathecolamines
myocardial
ischaemia
Fig. 2.
Integrated algorithm for the development of acute
pulmonary oedema in severe pre-eclampsia, and
a proposed unified theory of cardiac dysfunction of
the materno-foetal complex. SVR: systemic vascular
resistance, LVH: left ventricular hypertrophy, LV: left
ventricle, ESV: end-systolic volume, EDV: end-diastol-
ic volume, MPI: myocardial performance index, BNP:
brain natriuretic peptide.

















