CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 9/10, October/November 2013
e2
AFRICA
of the anterior mitral leaflet, interrupted everting sutures were
placed in the annulus and passed through the preserved posterior
leaflet.
The mitral valve was replaced with a 25-mm Sorin bileaflet
(Sorin Group
®
) mechanical prosthesis. All interrupted sutures
used for valve replacement were reinforced with Teflon (Bard
®
)
felt pledgets to prevent dehiscence of the prosthesis. The
aortic valve was replaced with a 19-mm Sorin (Sorin Group
®
)
mechanical prosthesis. The atrial wall and aorta were sutured and
the aortic cross-clamp was released.
The procedure was straightforward, but after separation from
cardiopulmonary bypass, the pericardium suddenly filled with
blood and, on lifting the heart, bleeding was identified from
the atrio-ventricular groove with swelling and haematoma in
the posterior wall of the left ventricle. The patient was placed
back onto bypass and cooled, and the heart was again perfused
with cold blood cardioplegia. Teflon (Bard
®
) felt-buttressed
interrupted sutures were placed, but considerable bleeding
continued.
We used BioGlue (Cryolife
®
) to stick a Teflon (Bard
®
) felt
patch approximately 5–6 cm over the area involved, but the
bleeding was not stopped, and the condition of the patient
deteriorated. All external repair methods were tried, but the
sutures themselves also caused additional damage to the friable
heart tissue. The patient died on the operating table.
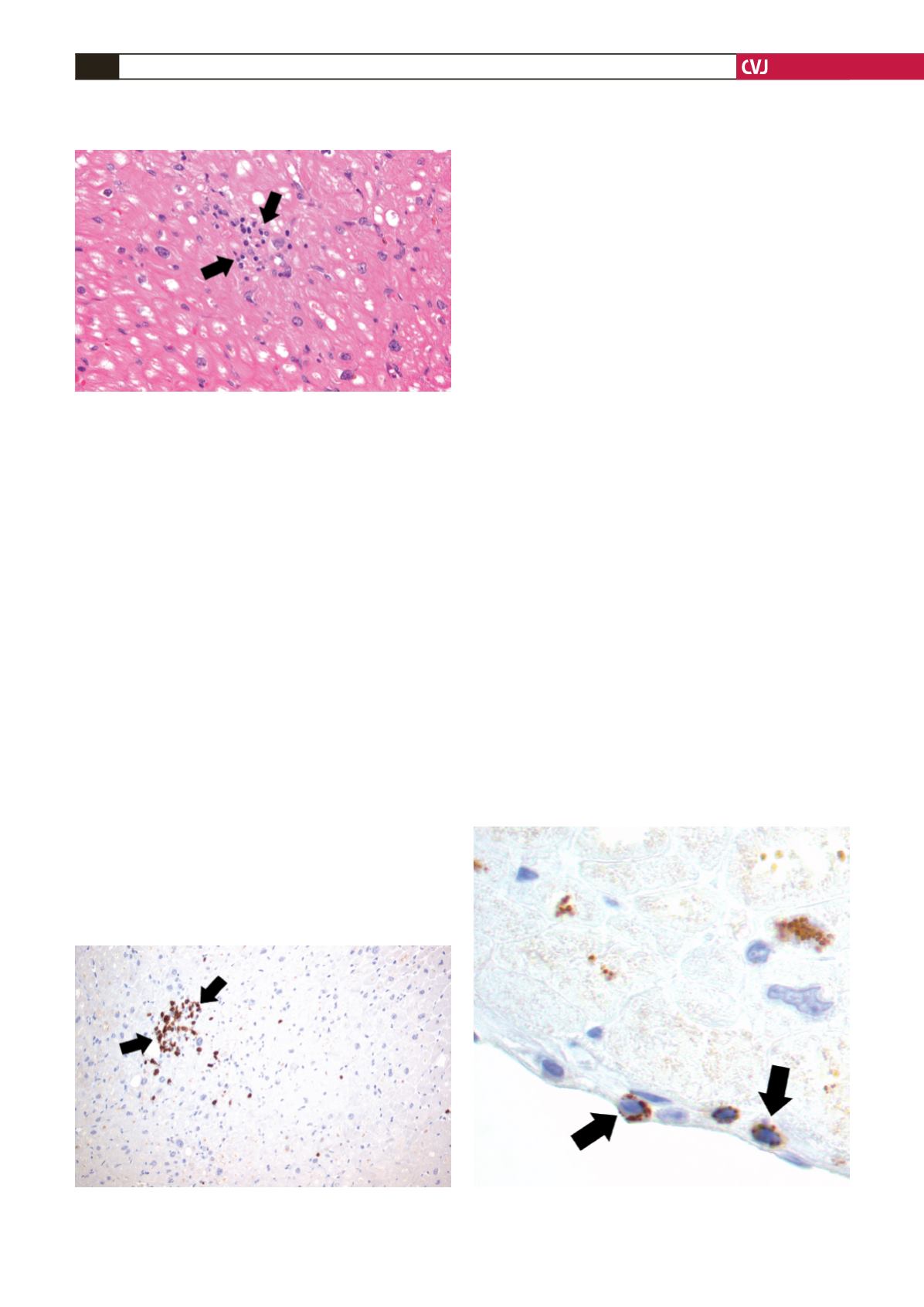
Due to the patients’ background we sent heart tissue for
biopsy. The biopsy showed diffuse myocardial necrosis with
infiltration by lymphocytes (Fig. 1). Immunohistochemistry
showed that the lymphocytes were mostly CD
3
+
T cells (Fig. 2)
and T-lymphocytes under the endocardium immune-expressing
cytotoxic TIA-1 enzyme (Fig. 3).
Discussion
Rupture of the left ventricular posterior wall, although infrequent,
is one of the most life-threatening sequelae of prosthetic
replacement of the mitral valve.
7
Its cause is controversial.
Female gender, advanced age, intrinsic myocardial disease,
mitral stenosis, small body size, and a small left ventricle have
been considered as predisposing risk factors.
8
A large number of
intra-operative factors that initiate the primary tear and cause the
transmural left ventricular rupture have been considered:
9
•
resection of excessive tissue during removal of the mitral
valve and consequent injury of the annulus
•
inaccurate sizing of the annulus and insertion of an oversized
prosthesis
•
entrance of deeply placed sutures into the ventricular myocar-
dium
•
apical venting of the left ventricle with dislocation of the heart
and consequent distortion of the left ventricular posterior wall
when a rigid mitral prosthesis is in place
•
forceful traction on the mitral annulus
•
mechanical injury to the ventricular endocardium caused by
such devices as metal vents and cardiotomy suckers, scissors
used during valve excision and retractors used to expose the
chordae tendineae
•
forceful compression of the ventricle against the prosthesis
during manual massage
•
positioning the strut of a bioprosthesis against the posterior
left ventricular wall.
In most cases it is difficult to define the causes of left
ventricular rupture. All the above intra-operative events have
been linked to left ventricular rupture, but none of these occurred
in the present case. In patients with a fragile and myxomatous
Fig. 1. Infiltration of cardiac muscle fibres by small
lymphocytes; a focal aggregation consisting of approxi-
mately 25 lymphocytes (H-E stain,
×
400).
Fig. 2. Detection of T-lymphocytes by CD
3
immunohisto-
chemical staining (
×
200).
Fig. 3. T-lymphocytes under the endocardium immuno-
expressing cytotoxic TIA-1 enzyme (
×
200).


