
CARDIOVASCULAR JOURNAL OF AFRICA • Volume 29, No 4, July/August 2018
AFRICA
211
36.1% had valvular heart disease and 13.4% had left ventricular
diastolic dysfunction (HFpEF: EF
>
50%).
The duration of follow up of the 150 participants ranged from
five to 180 days. After a median follow up of 90.5 days, 42 deaths
(cumulative mortality rate of 28%) were recorded. Equivalent
figures were five deaths (cumulative incidence 45.5%) in mild PH,
nine deaths (cumulative incidence 20.5%) in moderate PH and
28 deaths (cumulative incidence 29.5%) in severe PH (
p
=
0.28).
Discussion
Our study aimed at determining the prevalence, clinical profile
and mortality rate from PH in a rural setting in sub-Saharan
Africa. We noted a high prevalence of PH, late presentation to
healthcare facilities in an advanced state of heart failure, and
consequently a high mortality rate at six months of follow up.
These findings could be attributed to the poor socio-economic
status, hyper-endemicity of risk factors for PH, and limited
availability of PH-specific drug therapies. In the PAPUCO
study,
7
which was a multinational study on the epidemiology of
PH in Africa with recruitment centres mostly in urban areas,
similar findings were noted. Therefore it can be said that PH still
presents a challenge on the African continent overall and not
only in the rural setting.
Our observed prevalence of 15.6% is higher than the
average of 10% prevalence observed in Australia in 2012 and
in other European countries.
13
This is somewhat to be expected
considering the high burden of risk factors such as rheumatic
heart disease, schistosomiasis, tuberculosis, sickle cell disease
and HIV infection in sub-Saharan Africa, in addition to other
risk factors shared with high-income countries. In addition,
the SCC is located in a rural area that is difficult to access.
Therefore, patients are usually reluctant to visit the centre
until they are in advanced disease states or when referred by
cardiologists. A recent expert review on the global perspective
of the epidemiology of PH also supports our findings.
6
Among
the several co-morbidities assessed in our study population,
exposure to cooking fumes was the most common, especially in
women. This most likely results from the common practice in
Africa and Cameroon, particularly in the rural setting, where
women cook using open fires, unlike in high-income countries.
Systemic arterial hypertension was also common and in line with
studies from Africa,
7
USA
14,15
and Germany.
16
Hypertension is very common in sub-Saharan Africa where
it affects about 30% of the adult population, and mostly goes
undetected, undertreated and inadequately controlled.
17
It is the
principal cause of HF in sub-Saharan Africa. In the Pan-African
THESUS-HF registry of HF for instance, it was estimated that up
to 50% of HF cases were due to uncontrolled hypertension.
18
This
high prevalence of uncontrolled hypertension would most likely
also account for the high proportion of PHLHD in our study
population. With the growing epidemic of HF, LHD is now
globally recognised as the main cause of PH.
6,7,13
PHLHD was
dominated by patients with left ventricular systolic dysfunction,
while PH due to rheumatic valvular heart disease is still common
in our setting.
The clinical presentation was dominated by exertion
dyspnoea, fatigue, cough and palpitations, which are common
and non-specific symptoms in most patients with cardiovascular
and/or respiratory conditions. Study participants were slightly
overweight with a mean BMI higher than observed in a study
in Nigeria,
19
but lower than reported in the USA.
15
Most of
our participants presented with moderate to severe functional
limitation, with 70% of them presenting in WHO FC or New
York Heart Association (NYHA) class III and IV.
These findings are similar to those in the PAPUCO study,
7
and
to those of Baptista and colleagues in Portugal in 2013,
20
who
observed that 71% of their patients presented in WHO FC III
and IV, as well as those of Fikret and colleagues in Germany.
16
This global observation of late presentation to medical attention
could be explained by the fact that most symptoms and signs of
PH are non-specific and therefore cases are usually misdiagnosed
in primary care until the later stages when patients seek specialist
care. Furthermore, in Africa, poor access to healthcare, limited
availability of diagnostic tools for PH, and the general reluctance
of patients in rural settings to seek medical attention until the later
stages of illness could explain at least in part the late presentation.
About a third of our patients died within the first six months
of being diagnosed with PH. This mortality rate is three times
higher than that observed in the USA
15
and the UK.
9
The high
mortality rate in our setting is most likely accounted for to some
extent by the unavailability of disease-specific drug therapies.
The fact that patients present at an advanced stage of the disease,
and their inability to comply with follow-up visits reflects to
some extent their limited financial coping capacity, resulting in
death in the absence of adequate care.
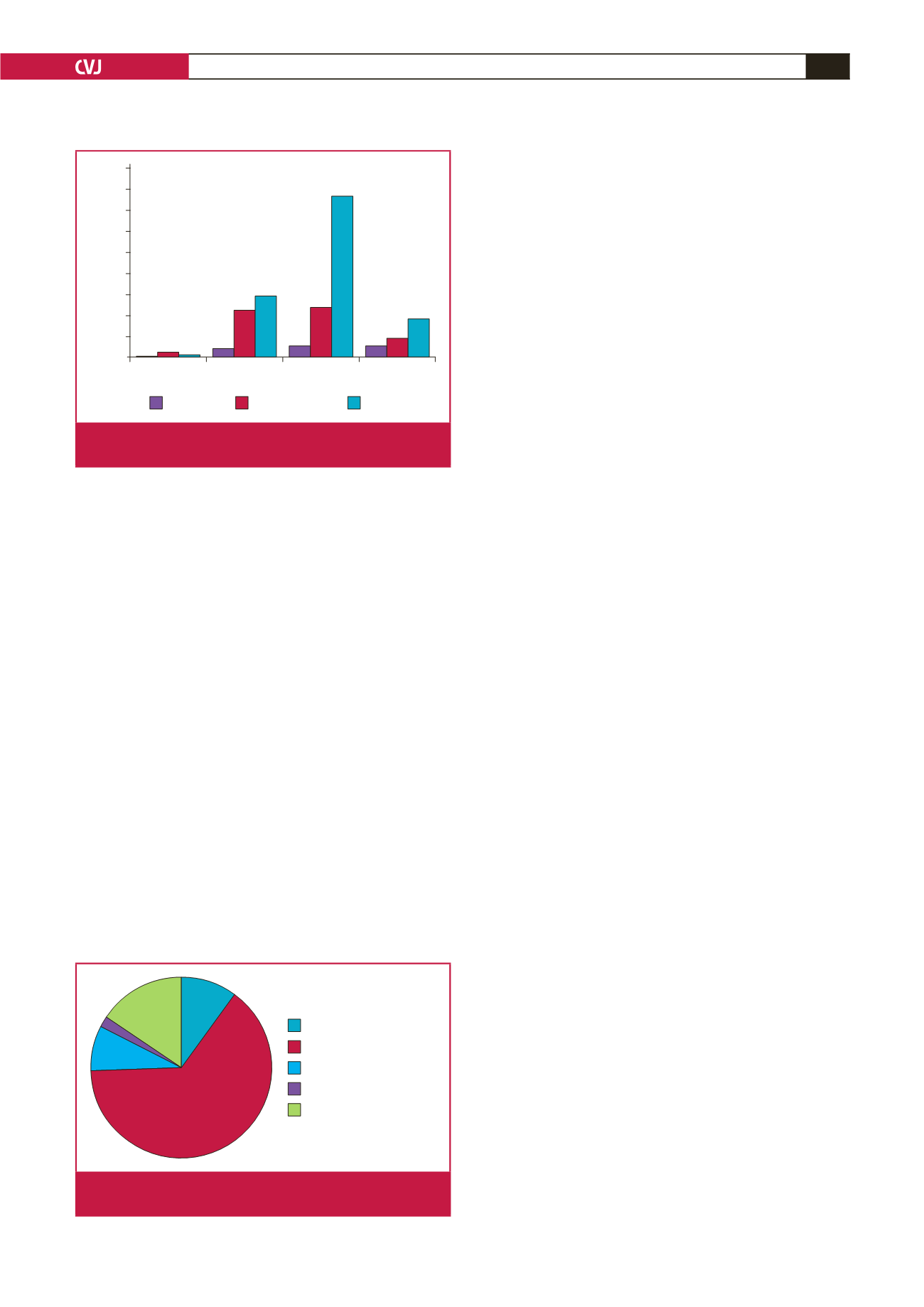
WHO FC
WHO FC I
WHO FC II
WHO FC III
WHO FC IV
Percentage
45
40
35
30
25
20
15
10
5
0
9.3
4.7
2.7
2.7
12
38.7
14.7
11.3
2
0.6 1.3 0
Mild PH
Moderate PH
Severe PH
Fig. 3.
Distribution of patients across WHO functional classes
and PH severity.
n
=
150
10%
64.7%
8%
15.3%
2%
Group 1 (PAH)
Group 2 (PHLHD)
Group 3 (PHLDH)
Group 4 (CTEPH)
Group 5 (PHUM)
Fig. 4.
Patient distribution according to the updated clinical
classification of PH.

















