
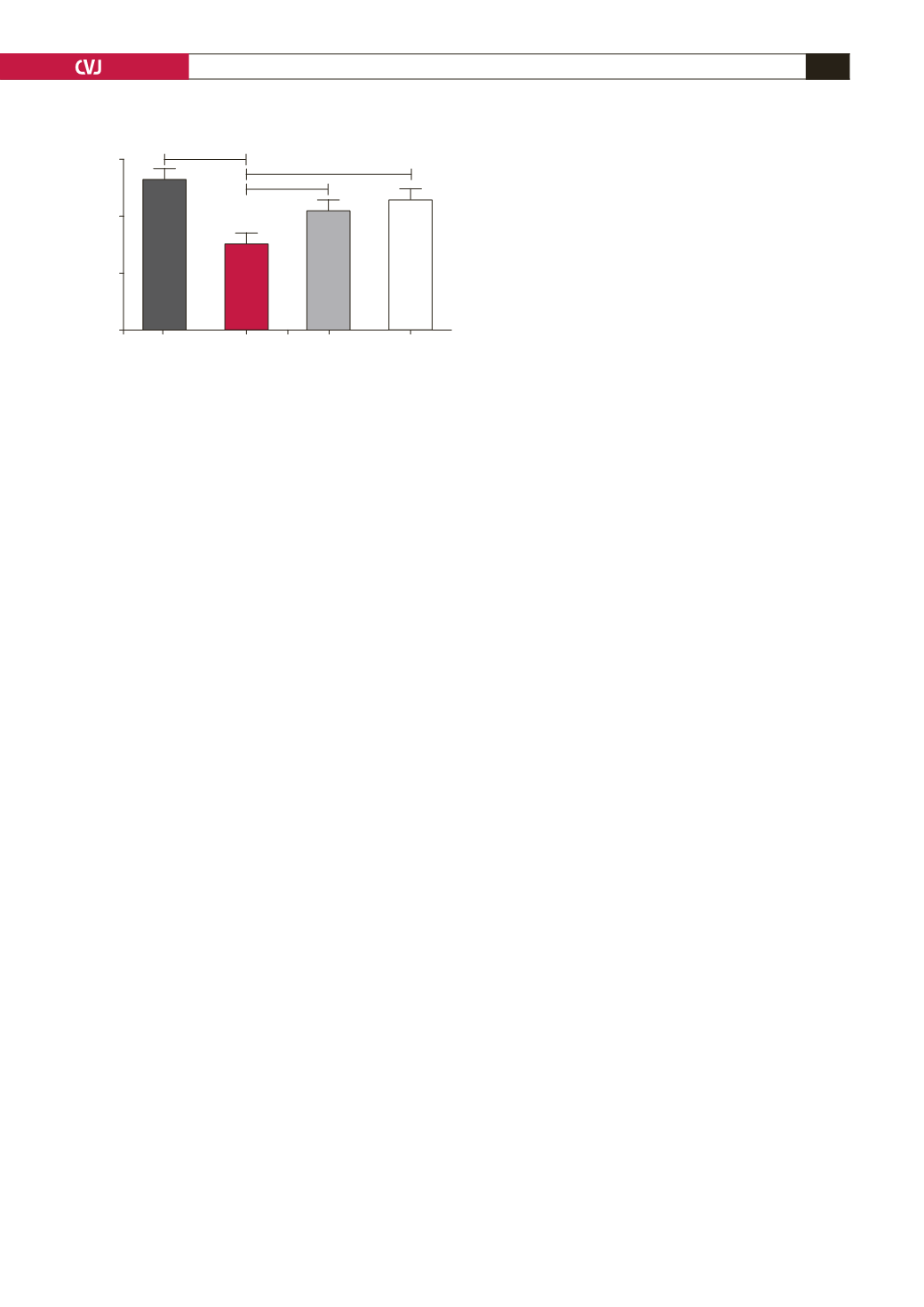
CARDIOVASCULAR JOURNAL OF AFRICA • Vol 24, No 2, March 2013
AFRICA
15
Captopril treatment elevated the urine output to 15
±
0.9 ml.
Treatment with
P
glandulosa
also elevated urine output to 13.68
±
0.80 ml (
p
<
0.01,
n
=
9 per group) (Fig. 7).
Discussion
Currently, the world is suffering from a silent epidemic starting
with obesity and culminating in type 2 diabetes.
3
Two of
the most debilitating complications of obesity, especially
centrally located obesity, responsible for the high morbidity and
mortality associated with such patients are hypertension and
heart disease.
19,20
In view of the need for effective medication
to supplement lifestyle changes to control these disease states,
utilisation of plant-based therapies are currently strongly
advocated.
7,21
Such therapies offer potentially cost-effective
management but need scientific validation of their effects.
Previous studies from our laboratory demonstrated that
the dried and ground pods of the
P
glandulosa
tree have a
potential benefit in the management of both type 1 and type 2
diabetes.
8
In view of the insulin-sensitising effects on isolated
cardiomyocytes from rats treated with
P
glandulosa
, we aimed
to determine whether this product has any cardioprotective or
anti-hypertensive effects.
In this study, we used three different animal models. The
first was a model of pre-diabetes (DIO), as also indicated by
the biometric data presented in Table 1. These animals were
fed an obesity-inducing diet containing only 16% fat.
11,13
DIO
animals become insulin resistant but not diabetic, as the blood
glucose levels never rose above ~ 6.5 mmol/l. This was however
significantly higher than the levels found in the control, chow-
fed animals. In order to keep the blood glucose levels low, the
animals presented with high plasma insulin concentrations.
Although
P
glandulosa
treatment did not significantly
alter these parameters, the clinically important two-hour blood
glucose values after a glucose tolerance test were significantly
higher in the DIO animals and were effectively lowered by the
treatment (Fig. 1). This underscores the slight effect on blood
glucose handling previously reported.
8
Determination of infarct size in
ex
vivo
perfused rat hearts
as a measure of myocardial damage incurred by ischaemia
followed by reperfusion, is taken as the gold standard to prove
cardioprotection.
15
We previously showed that hearts from the
DIO rats developed larger infarct sizes when subjected to
regional ischaemia followed by reperfusion.
10
After eight weeks of treatment of DIO rats or CIRKO mice
with
P
glandulosa
, it was clearly demonstrated that there was an
infarct-sparing effect elicited by ingestion of this plant material
(Figs 2, 3). As the CIRKO mice do not possess a myocardial
insulin receptor, the protection found in these animals confirmed
the results obtained in the rat model and underscores that
protection does not occur via the insulin-secretory effects of
P
glandulosa
, as previously reported.
8
One of the best-described and researched mechanisms of
protection of the heart against ischaemia–reperfusion injury and
infarction is activation of the PI-3K, PKB/Akt pathway, normally
activated by various extracellular substances.
22-24
Activation
of this pathway has several anti-apoptotic effects, leading to
limitation of the development of an infarct after ischaemia.
In addition, activation of PKB/Akt is a pre-requisite for glucose
uptake by the heart.
25
Myocardial glucose is taken up via the two
transporters Glut 1 and Glut 4. An improved ability to import and
utilise glucose is cardioprotective when the heart is subjected to
the absence of oxygen, as induced by ischaemia. The heart then
uses the energy generated by glycolysis to protect itself.
Measurement of the expression of both Glut 1 and Glut 4
showed no differences between hearts from control and DIO rats.
However, the lower ratio of phosphorylated to total protein of
PKB/Akt found in hearts from the DIO animals may have been
detrimental during an ischaemic incident. In addition, there was
lower expression of the p85 subunit of PI-3K documented in
these hearts, which may have exacerbated this effect.
Both of these detrimental changes were improved by
P
glandulosa
treatment. The changes documented in the
phosphatase PTEN will further the positive effects found in
both PI-3K and PKB/Akt as the lower expression and elevated
phosphorylation of this enzyme will elevate the activity of PKB/
Akt when the latter is stimulated.
18
PTEN normally inactivates
PKB/Akt.
17
These changes may play a central role in the
protection that
P
glandulosa
treatment confers on the heart.
The second rat model was aimed at specifically inducing the
development of hypertension. A modification of a high-fat diet
was used (HFD).
12
These animals, in contrast to the DIO animals,
developed severe hypertension within a four-week period, as
shown in Fig. 6A and B. Not only was
P
glandulosa
treatment
able to prevent the development of hypertension when given in
conjunction with the high-fat diet, but it normalised elevated
blood pressure within two weeks.
The hormonal effects associated with a high-fat diet in rats,
namely elevated vasopressin as well as activation of the renin–
angiotensin system, leading to elevated aldosterone levels may
both be involved in the development of hypertension in these
animals.
26-28
Vasopressin, the anti-diuretic hormone leads to water
retention and therefore the development of high blood pressure.
In addition, it is associated with vasoconstriction.
28
Similar effects
can be expected from elevated sympathetic activity, leading to
elevated aldosterone levels. Measuring the 24-hour urine output
of the HFD animals underscored this, as the HFD animals had a
significantly lower urinary output than the controls.
According to Lee and Blaufox,
29
a volume of 16–17 ml urine
can be expected from normal animals in the weight range of our
experimental rats (control 258.49
±
15.03 vs HFD 327
±
12.90
g,
p
<
0.05,
n
=
14 per group) while a high-fat diet will result in
concentration of this volume, indicating water retention. It can
also be speculated that, in parallel with the latter effect, there will
be vasoconstriction, contributing to the observed hypertension.
Fig. 7. Rats on the high-fat diet were individually placed
in metabolic cages for the collection of urine over a
24-hour period. Data were collected at 12 weeks after the
diet was started. **
p
<
0.01, ***
p
<
0.001,
n
=
9 per group.
20
15
10
5
0
Control
HFD
HFD + P Captopril
ml urine
**
***
**



















